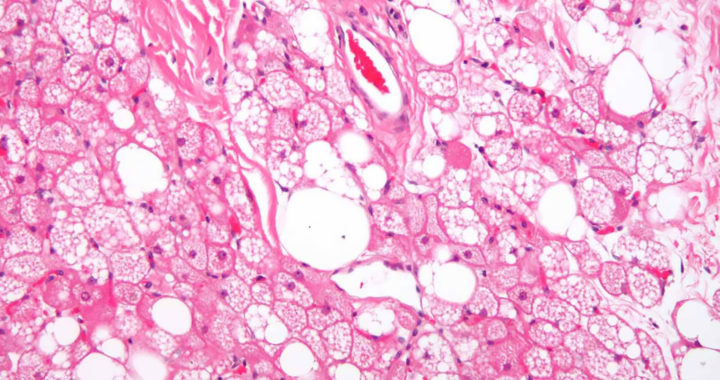
Brown adipose tissue or brown fat is one of the two types of fat or adipose tissue abundant in hibernating animals and newborn humans. Compared to white adipose tissue or white fat, which contains a single lipid droplet, brown fat contains numerous smaller lipid droplets and a higher number of iron-containing mitochondria.
It is worth mentioning that scientists initially thought that the function of brown adipose tissue in adult humans is negligible. However, several studies revealed that it plays a critical role in metabolism, especially in the consumption of energy.
Brown Fat as a Solution to Obesity and Raising Metabolism: A Review of Studies
Understanding the Role of Brown Adipose Tissue
This specialized tissue is responsible for non-shivering thermogenesis—a process of generating heat and raising body temperature by burning calories instead of shivering.
In their study that identified B cell factor-2 or Ebf2 as a protein responsible for the development, differentiation, and function of fat cells, researchers Sona Rajakumari et al. explained that brown fat burns excess energy while white fat stores them.
Rajakumari et al. performed an experiment that involved overexpressing the Ebf2 protein in precursor white fat cells to mature into brown fat cells, Rajakumari et al. further demonstrated the difference between the two types of adipose tissue. The induced brown adipose tissue accordingly consumed higher amounts of oxygen, had a greater number of mitochondria, and had an increased expression of genes involved in heat production.
While brown adipose tissue is abundant in children, its amount decreases as they transition to adolescence and further into adulthood. Researchers Lindsay Robinson et al. mentioned that most adults only have 50 to 100 grams of brown fat. However, the capacity of this tissue to generate is 300 times greater than other tissues in the body.
Combatting Obesity by Exploiting Brown Fat
Because brown adipose tissue burns calories at rest, researchers are exploring the role of this tissue in weight loss and in preventing obesity.
Researchers Laurie Goodyear et al. noted that white fat is associated with increased body mass and obesity. On the other hand, because brown adipose tissue is associated with lower body mass index and high-energy consumption using glucose and fatty acids as fuel, they believe that it plays a fundamental role in the maintenance of a leaner and a more metabolically healthy phenotype. They also hypothesized that a brown fat transplant could be used as a therapeutic tool to combat obesity and metabolic disease.
To test their hypothesis, Goodyear et al. performed brown fat transplants in mice that were further fed with either a normal diet or a high-fat diet. After eight to 12 weeks of transplantation, recipient mice had demonstrated improved glucose tolerance, increased insulin sensitivity, lower body weight, decreased fat mass, and a complete reversal of insulin resistance induced by a high-fat diet. The transplanted brown adipose tissue also secreted several hormones, including IL-6, which mediated the body throughout the body.
Brown fat transplants could be a possible solution to manage weight and combat obesity. However, the separate studies of Rajakumari et al. and Junko Sugatani et al. also suggest the use of protein targeting through drug therapy.
Take note that the study of Rajakumari et al. identified Ebf2 protein as responsible for the development and maintenance of brown fat cells. However, the researchers reminded that this protein is not a readily druggable target. They still suggested that it is possible to pharmacologically block or stimulate the interaction of Ebf2 with a partner protein.
Sugatani et al. also identified platelet-activating factor receptors or PAFR gene deficiency in the development of obesity due to impaired thermogenesis activity. In an experiment that involved knocking down the PAFR gene in mice, they found out that the deficiency resulted in brown fat dysfunction as characterized by impairment of thermogenesis function.
Cold Climates and Brown Adipose Tissue
Other studies also suggest the use of natural mechanisms to stimulate the energy-consuming activity of brown adipose tissue. For example, A. C. Carpentier et al. enrolled six healthy adult men in a study that involved controlled cold exposure conditions.
All subjects demonstrated substantial non-esterified fatty acid and glucose uptake upon cold exposure. The findings also demonstrated cold-induced activation of oxidative metabolism in brown fat but not in adjoining skeletal muscles and adjoining subcutaneous adipose tissue. This activation was associated with an increase in total energy expenditure.
However, the researchers also found that exposure to warm temperatures did not result in similar energy expenditure and activations.
The findings of Carpentier et al. are also echoed in another study by Paul Lee et al. that involved enrolling five healthy adult men and subjecting them to temperature acclimation that lasted for four months.
Results of the study revealed that long-term exposure to cold environments can stimulate brown fat growth and activity by about 30 to 40 percent. Prolonged exposure to warm climates decreased the amount of this tissue below that of baseline.
Tore Bengtsson et al. explained how a cold environment activates brown adipose tissue. Accordingly, when the body encounters cold temperatures, the sympathetic nervous system activates adrenoceptors on the surface of brown fat cells to stimulate glucose uptake from the bloodstream. Brown fat cells then use this glucose as a fuel source to generate body heat.
An accompanying commentary on the study of Carpentier et al. mentioned that increasing the amount of brown adipose tissue in a person is unlikely to make an individual leaner. The trick is to make sure that this tissue is active and burns calories.
Impact of Stress on Brown Fat Activity
Stress also appears to induce brown fact activity. Researchers Labros S. Sidossis et al. studied burn trauma patients. They initially hypothesized that burn trauma provides a unique model of severe and prolonged stress in which adrenaline release is massively increased for several weeks following the injury.
After enrolling 72 patients that had sustained severe burns over approximately 50 percent of their bodies and a comparison group composed of 19 healthy individuals, the researchers took samples of white fat. They measured the metabolism of the samples and the composition of the fat cells, while also taking note of the resting metabolic rate of the participants. Take note that they specifically took samples of white fat in burn trauma patients at different time points following their injury.
Findings revealed that in burn trauma patients, there was a gradual shift in molecular and functional characteristics of white fat to a more brown fat phenotype over time. This suggested the progressive browning of white fat in response to a burn injury.
Sidossis et al. further explained stress can result in the browning of white fat. Accordingly, brown fat cells express a protein called UCP1. This protein prompts mitochondria to burn calories without making any chemical energy—just heat. Adrenaline from stress due to burn trauma activates this protein. They concluded that the browning of white fat is possible and that their study can help pave the way for the development of drugs that can mimic the effects of burn trauma.
But severe adrenergic stress is not the only mechanism for stimulating brown fat. Mild tress can directly stimulate the activity of existing brown fat. Robinson et al. enrolled five healthy lean women. They subjected them to short math tests in the first run and had them watch a relaxation video in the second run. To assess stress response, they measured cortisol levels in the saliva. On the other hand, to measure brown fat activity, the researchers used infrared thermography to detect changes in the temperature of the skin overlying the main area of brown fat.
Although the actual mathematics tests did not elicit an acute stress response, the anticipation of being tested did and led to raised cortisol and warmer brown fat. Both were positively correlated, with higher cortisol linked with more fat activity and thus more potential heat production.
The researchers concluded that their study might open new techniques to exploit brown adipose tissue for weight management and combatting obesity. These techniques would involve inducing mild stress and incorporating them into dietary and/or environmental intervention programs.
FURTHER READINGS AND REFERENCES
- Rajakumari, S., Wu, J., Ishibashi, J., Lim, H.-W., Giang, A.-H., Won, K.-J., Reed, R. R., and Seale, P. 2013. “EBF2 Determines and Maintains Brown Adipocyte Identity.” Cell Metabolism. 17(4): 562-574. DOI: 1016/j.cmet.2013.01.015
- Robinson, L. J., Law, J. M., Symonds, M. E., and Budge, H. 2016. “Brown Adipose Tissue Activation as Measured by Infrared Thermography by Mild Anticipatory Psychological Stress in Lean Healthy Females.” Experimental Physiology. 101(4): 549-557. DOI: 1113/ep085642
- Stanford, K. I., Middelbeek, R. J. W., Townsend, K. L., An, D., Nygaard, E. B., Hitchcox, K. M., Markan, K. R., Nakano, K., Hirshman, M. F., Tseng, Y.-H., and Goodyear, L. J. 2012. “Brown Adipose Tissue Regulates Glucose Homeostasis and Insulin Sensitivity.” Journal of Clinical Investigation. 123(1): 215-223. DOI: 1172/jci62308
- Sugatani, J., Sadamitsu, S., Yamaguchi, M., Yamazaki, Y., Higa, R., Hattori, Y., Uchida, T., Ikari, A., Sugiyama, W., Watanabe, T., Ishii, S., Miwa, M., and Shimizu, T. 2013. “Antiobese Function of Platelet‐Activating Factor: Increased Adiposity in Platelet‐Activating Factor Receptor‐Deficient Mice with Age.” The FASEB Journal. 28(1): 440-452. DOI: 1096/fj.13-233262