Researchers at Oregon Health and Science University have engineered a striking proof of concept. They were able to convert human skin cell nuclei into functional egg cells that can be fertilized. The advance, called mitomeiosis, produced a small number of embryos in vitro. Their findings are discussed in a paper published on 30 September 2024 in Nature Communications.
An Experimental Cell Division Process Called Mitomeiosis Enabled Scientists to Convert Skin Cells Into Egg Cells
From Stem Cells to Somatic Transfer
Experiments in mice proved that egg creation outside the body was possible, but human cells resisted similar attempts, often stalling or collapsing in early stages.
For decades, banking on the promise of assisting individuals with infertility and advancing further fundamental biology, scientists have searched for ways to generate human eggs outside the body. Earlier efforts often focused on reprogramming adult cells into pluripotent stem cells and guiding them through lengthy developmental pathways.
Mouse studies provided initial evidence that gametes might be created artificially, but translating those successes to humans proved difficult. Attempts to coax stem cells into eggs frequently stalled at immature stages or produced abnormal chromosomes. This highlights the complexity of meiosis and the sensitivity of reproductive development.
The OHSU team pursued an alternative route by adapting somatic cell nuclear transfer, historically used in cloning research, and merging it with mechanisms that mimic meiotic division. Their goal is not immediate clinical use but to stumble upon discoveries that could transform treatment options and expand reproductive possibilities in the future.
Inventing the Hybrid Cell Division Process Called Mitomeiosis
The nucleus of a skin cell was carefully placed inside the emptied egg, but this creates a mismatch in chromosome count that demanded a biological reset.
Lead researcher Shoukhrat Mitalipov and first author Nuria Marti-Gutierrez, together with the rest of the team, used two main components. These were donated human eggs and skin cells containing complete sets of chromosomes. They first removed the nucleus from each egg. This step cleared space for a replacement nucleus that would supply the genetic material.
The next step involved extracting the nucleus from a skin cell and inserting it into the enucleated egg. At this stage, it is worth mentioning that the egg cell held the wrong amount of DNA, because skin cells normally carry 46 chromosomes instead of the 23 chromosomes expected in a mature reproductive cell. The researchers needed to correct this mismatch.
A combination of electrical stimulation and a chemical agent called roscovitine was used to give the egg cell the correct number of chromosomes. These serve as signals to encourage the egg to behave as though it were undergoing meiosis. Note that meiosis is a type of cell division process responsible for halving chromosome numbers during egg development.
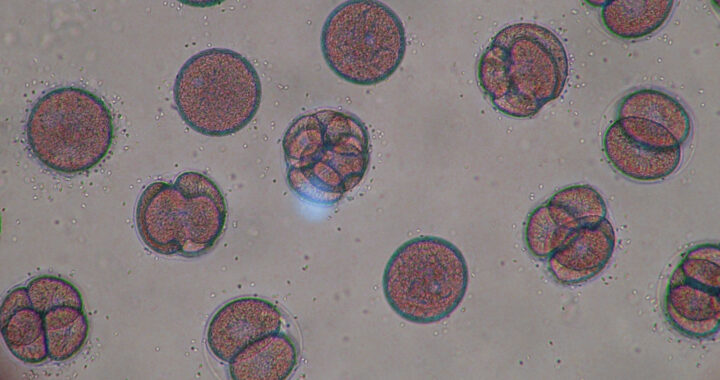
The team described the resulting sequence as mitomeiosis because it is a hybrid of ordinary cell division and reproductive division. Hence, once the egg cell discarded half its chromosomes, it functioned like a natural oocyte. Researchers then fertilized several of these engineered eggs with sperm to test whether they could begin embryonic development.
Experiment and Findings Suggest a Mere Proof of Concept
Scientists monitored each engineered egg after fertilization to determine whether it could behave like a naturally produced oocyte under standard lab conditions.
Researchers created 82 egg cells using the mitomeiosis approach and then fertilized them with sperm cells through standard laboratory methods. Each engineered egg cell was monitored to see whether it could begin dividing into an embryo. This is essential to understand and determine if the approach can be used in regular in vitro fertilization procedures.
Most of those fertilized eggs stopped developing very early at only a few rounds of cell division before arresting entirely. Many showed chromosomal problems, including extra copies, missing segments, or uneven distributions of genetic material, all of which interfered with normal growth and blocked any progression beyond the earliest stages.
Only around 9 percent of the eggs, or fewer than ten out of the entire batch of the engineered egg cells, reached the blastocyst stage after six days in culture. Take note that this is the point when an inner cell mass begins forming the earliest building blocks of an embryo. But none of the eggs were able to continue developing beyond that carefully regulated limit.
Further genetic analyses revealed another limitation. The chromosomes from the skin cell nucleus did not pair or separate as neatly as they would during natural egg formation. The process also did not create the genetic mixing seen in normal reproduction. This reinforces the fact that the experiment and the approach remain a proof of concept.
Important Promises and Implications
The experimental technique is a novel approach that offers a provocative alternative to stem cell methods and forces scientists and society to confront what comes next.
The experiment and subsequent findings overturn a long-held belief that body or specialized tissue cells are locked into their identities forever. Specifically, by forcing a skin cell nucleus to behave like an egg, the researchers showed the principle that reproductive potential can be reassigned and incited with the right environment and chemical triggers.
It is also important to note that the approach differs from stem-cell-based strategies that require reprogramming and gradual transformation. Skipping these steps and using nuclear transfer paired with chromosome reduction resulted in a novel but experimental approach that hints at a faster path to producing or engineering egg cells outside the body.
The findings also open new conversations about fertility futures, oversight, and ethics. Scientists emphasize that the work is not ready for clinical use, but its boldness ensures more debate, closer scrutiny, and increased pressure to define how such approach and other relevant technologies might eventually be regulated for more practical applications.
FURTHER READING AND REFERENCE
- Marti Gutierrez, N., Mikhalchenko, A., Shishimorova, M., Frana, D., Van Dyken, C., Li, Y., Ma, H., Koski, A., Liang, D., Lee, S.-G., Eyberg, D., Safaei, Z., Kang, E., Lee, Y., O’Leary, T., Lee, D., Krieg, S., Wu, D., Rubin, E., … Mitalipov, S. 2025. “Induction of Experimental Cell Division to Generate Cells with Reduced Chromosome Ploidy.” Nature Communications. 16(1). DOI: 1038/s41467-025-63454-7